Don't be salty: how to make desalination work in tomorrow's world
Although water is often scarce for human consumption and agriculture, this planet is three-quarters covered by water. The problem is getting the salt out, and that's normally done by the Earth's water cycle, which produces rain and similar phenomena that replenish the amount of fresh water. About 3% of the water on Earth is fresh water, a fraction of which is drinking water.
Over the past few decades, the use of desalination has increased year on year, especially in countries like Saudi Arabia, Israel and the United Arab Emirates, but parched US states like California are turning more and more towards desalination technologies. The obvious hurdles desalination faces – regardless of the exact technology used – involve the energy required to operate these systems and the ultimate cost of producing potable water versus importing it from elsewhere.
Other issues that arise with desalination include environmental impact, particularly brine waste and possibly marine life sucked into the intake pipes. As the need for desalination increases, what options are available to reduce energy requirements and environmental impact?
Heating, filtering and waste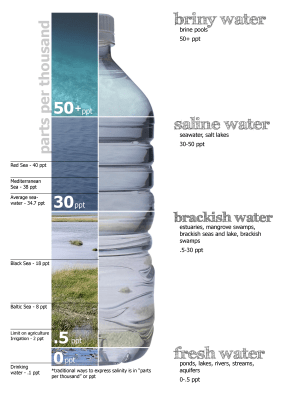 Water salinity chart. (Credit: Peter Summerlin)
Water salinity chart. (Credit: Peter Summerlin)A common type of desalination is distillation, which is essentially what also happens in nature through the evaporation of surface water. As water is heated, it evaporates, leaving salts and other dissolved solids. When this process is done using intense heat and in stages, it is called multi-stage flash distillation (MSF), which is one of the three most common types of distillation, along with multi-effect distillation (MED) which uses stages with steam heating which couples to the next stage, effectively reusing the heat. However, by far the most common type of distillation (~69% share) is reverse osmosis (RO), which uses a pressure differential across a membrane that allows water molecules to pass, but not molecules. salt molecules and many other dissolved solids.
It is important to keep in mind that the result of none of these large-scale desalination processes is a clean separation between water and whatever remains. Instead, there is a freshwater outlet (~40% for reverse osmosis), with a concentrate flow that is essentially brackish water, allowing with all the contaminants found in saline water or intake brackish. This concentrate flow is what is returned to the sea or other body of water from which the intake water was drawn.
In addition to the much higher salt content of this concentrate stream, about twice that of seawater, it also has a much higher temperature...
Although water is often scarce for human consumption and agriculture, this planet is three-quarters covered by water. The problem is getting the salt out, and that's normally done by the Earth's water cycle, which produces rain and similar phenomena that replenish the amount of fresh water. About 3% of the water on Earth is fresh water, a fraction of which is drinking water.
Over the past few decades, the use of desalination has increased year on year, especially in countries like Saudi Arabia, Israel and the United Arab Emirates, but parched US states like California are turning more and more towards desalination technologies. The obvious hurdles desalination faces – regardless of the exact technology used – involve the energy required to operate these systems and the ultimate cost of producing potable water versus importing it from elsewhere.
Other issues that arise with desalination include environmental impact, particularly brine waste and possibly marine life sucked into the intake pipes. As the need for desalination increases, what options are available to reduce energy requirements and environmental impact?
Heating, filtering and waste Water salinity chart. (Credit: Peter Summerlin)
Water salinity chart. (Credit: Peter Summerlin)A common type of desalination is distillation, which is essentially what also happens in nature through the evaporation of surface water. As water is heated, it evaporates, leaving salts and other dissolved solids. When this process is done using intense heat and in stages, it is called multi-stage flash distillation (MSF), which is one of the three most common types of distillation, along with multi-effect distillation (MED) which uses stages with steam heating which couples to the next stage, effectively reusing the heat. However, by far the most common type of distillation (~69% share) is reverse osmosis (RO), which uses a pressure differential across a membrane that allows water molecules to pass, but not molecules. salt molecules and many other dissolved solids.
It is important to keep in mind that the result of none of these large-scale desalination processes is a clean separation between water and whatever remains. Instead, there is a freshwater outlet (~40% for reverse osmosis), with a concentrate flow that is essentially brackish water, allowing with all the contaminants found in saline water or intake brackish. This concentrate flow is what is returned to the sea or other body of water from which the intake water was drawn.
In addition to the much higher salt content of this concentrate stream, about twice that of seawater, it also has a much higher temperature...
What's Your Reaction?





















